Identification of exploitable vulnerabilities in cancer
Targeting deregulated protein stability in cancer biology
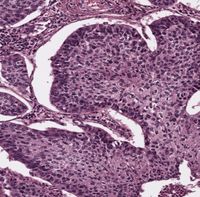
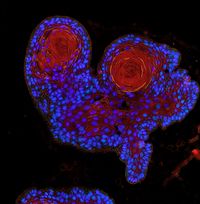
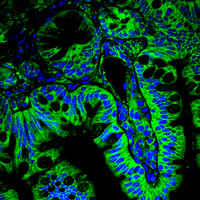
Our research focus is the deregulation of protein turnover as a central driver in tumourgenesis, using state-of-the-art ex vivo and in vivo tools.
Spatial control of protein stability is a key requirement to establish and maintain the complex interplay of various cell types in organs and is a guarantor for tissue homeostasis. Loss of control over tissue maintenance and repair by aberrant accumulation of transcription factors can give rise to uncontrolled proliferative cells, a precursor stage of a fatal disorder, cancer.
One central mechanism tumour cells alter during oncogenic transformation is the ubiquitin proteasome system (UPS), which is regulating virtually all biological processes, including, but not limited to, DNA damage, senescence, cell cycle, DNA replication and the immune system.
Despite the prominent involvement of the UPS in cancer, our understanding of how tumour cells alter the UPS system very early in transformation is rather limited, but has the potential to hold novel therapeutic strategies. Targeting of common essential pathways and exploiting tumour intrinsic vulnerabilities holds the potential to not only improve current treatment for late stage, but also for early stage patients.
By combining state of the art techniques, such as genetically tailored in vivo tumour models recapitulating the human disease, murine and patient derived organoids, as well sequential gene perturbation in vivo assays, we aim at identifying and validating novel vulnerabilities in cancer.
More about our RESEARCH
Research
News
Selected Publications
Team Members